Recognized for its versatility and potential as a clean energy carrier, hydrogen is poised to address the energy requirements of various sectors, including heavy industry, transportation, and power generation.
Evolution of Raw Materials in Green Hydrogen Production: A Comparative Analysis of Current Trends and Technologies
Dr. Raj Shah and Angelina Mae Precilla | Koehler Instrument Company
1. Introduction
The world is currently grappling with a critical energy crisis, driven by a surging global population and escalating energy demands. This escalating demand is predominantly met by fossil fuels—coal, oil, and gas—which not only strain energy resources but also significantly contribute to greenhouse gas emissions (GHG), exacerbating environmental concerns and climate change. Fossil fuels are the main contributors to climate change, responsible for over 75% of global greenhouse gas emissions [1]. Burning these fuels releases carbon dioxide into the air, trapping heat and causing global warming. This persistent reliance on fossil fuels intensifies concerns about environmental degradation and climate change, compelling a global shift toward sustainable and carbon-neutral energy solutions. In response to these challenges, there is a growing global impetus to transition towards such solutions, as highlighted by international agreements such as the 2015 Paris Agreement, which aims to limit the rise in global temperature and mitigate the adverse effects of climate change [2].
Amid this urgent need for decarbonization, hydrogen has emerged as a key player in the quest for sustainable energy. Recognized for its versatility and potential as a clean energy carrier, hydrogen is poised to address the energy requirements of various sectors, including heavy industry, transportation, and power generation [3]. Its diverse production methods, including renewable sources, and its application in fuel cells for vehicles and industrial processes, position hydrogen as a promising solution to reduce carbon emissions and facilitate the transition towards a more sustainable energy future [4].
The global recognition of hydrogen's significance is evident in the adoption of national hydrogen strategies by more than 25 countries, with the European Union actively pursuing hydrogen as a key element of its strategy to achieve carbon neutrality by 2050 [5, 6]. Notably, many of these strategies prioritize the production of green hydrogen, which is generated through the electrolysis of water using renewable energy sources, offering a promising avenue for addressing the energy crisis while producing zero greenhouse gas emissions [7].
As governments, industries, and investors increasingly acknowledge the importance of transitioning to renewable energy sources, there is an increase in interest and investment in green hydrogen technology. Research and development efforts are underway to drive down costs, improve efficiency, and scale up production, although challenges such as cost competitiveness, infrastructure development, and technological advancements still need to be addressed to unlock the full potential of green hydrogen.
This review will provide a comprehensive overview of hydrogen production, including the different types of hydrogen technology and the challenges in its production, such as elevated costs and the lack of an existing infrastructure. It will also address how recent advancements in green hydrogen technology are poised to overcome these challenges. Furthermore, the review will examine the evolution of raw materials in green hydrogen production and explore the geographic variations in its implementation, offering valuable insights into the opportunities and challenges associated with the widespread adoption of this environmentally friendly energy source.
2. Hydrogen production
The current state of hydrogen production is dominated by fossil fuel-based methods, in almost 900 million metric tons of CO2 annually [8]. Steam methane reforming (SMR) and coal gasification are the primary methods, both being energy-intensive and highly polluting. On the other hand, electrolysis, a cleaner but less developed technology, contributes only 2% to total production. The limited use of electrolysis is largely due to its inefficiency; 180 MJ of energy is required to produce 1 kg of H2, which has an energy content of 143 MJ [9]. However, with the use of renewable energy sources, there is the potential to produce hydrogen with no carbon footprint.
2.1. Color-coded hydrogen types
Hydrogen is designated with specific colors based on how it is produced [7, 10]. Among the hydrogen types, gray and brown hydrogen are significant contributors to GHG emissions, while blue hydrogen uses carbon capture techniques to ensure no CO2 is emitted into the atmosphere. Green hydrogen, produced from renewable sources, aims for zero emissions. Other less common colors include turquoise, purple, pink, red, and white hydrogen, each produced through different methods such as methane pyrolysis, nuclear power, and high-temperature catalytic water splitting.
A detailed comparison of different hydrogen production technologies is presented in Table 1, highlighting their respective strengths, weaknesses, and efficiencies. This analysis positions green hydrogen as the most promising option, emphasizing its capacity for zero emissions during both production and utilization, facilitated by the utilization of renewable energy sources. The choice between blue and green hydrogen hinges on factors such as cost, resource availability, and environmental goals.
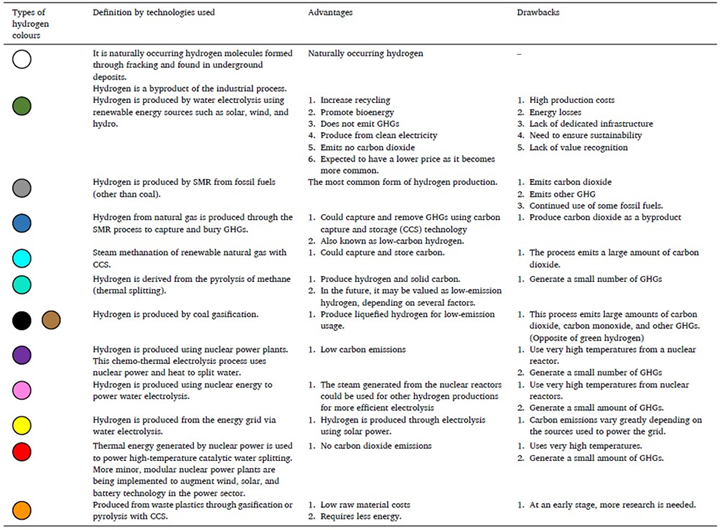
Table 1. Different colors of hydrogen defined by the technologies used [9].
2.2. Green hydrogen
Green hydrogen is a form of hydrogen produced using only renewable energy sources, ensuring zero carbon emissions throughout its life cycle [11]. It is derived from water through a process called electrolysis, where water is split into its constituent elements, gaseous hydrogen, and oxygen. The electrolysis process relies solely on electricity generated from renewable sources such as wind, water, or solar, ensuring that the energy input is clean and free from greenhouse gas emissions. This results in the entire lifecycle of green hydrogen, from production to utilization, being characterized by minimal environmental impact [12].
Green hydrogen carries great potential for energy storage because it can be burned when required without any carbon dioxide emissions or used to convert back to electricity when needed. It can be produced using surplus renewable electricity generated during off-peak periods, making it an efficient means of harnessing renewable energy. Hydrogen allows vast quantities of clean energy to be stored for long durations, making it a viable option for seasonal energy storage and electrical grid support during non-peak production hours.
One significant environmental benefit of green hydrogen lies in its potential to decarbonize challenging sectors such as heavy industries [3]. Industries like steel and cement production rank among the major contributors to climate-warming emissions. These sectors demand high-energy-density fuel or intense heat, making the transition away from fossil fuels difficult. Green hydrogen, however, offers a solution by being produced wherever there is water and electricity, and it can be utilized on-site or transported to other locations. Unlike batteries, green hydrogen can be generated from excess renewable energy and stored in large quantities for an extended duration, positioning it as the most practical energy carrier for future energy systems (Figure 1) [13].

Figure 1. Transition from fossil-based to hydrogen-based energy [12].
Furthermore, green hydrogen can be transformed into electricity or synthetic gas and used for commercial, industrial, or mobility purposes [14]. It is also easily storable, making it a versatile energy carrier. Moreover, green hydrogen can help manage the intermittency of renewable energy sources like wind and solar. It can be produced when these sources are abundant and stored for use when they are not, effectively smoothing out the fluctuations in renewable energy production.
The increasing demand for sustainable energy carriers and chemical industry feedstocks has fueled the rapid growth of green hydrogen technologies. Four major approaches—water electrolysis, photoelectrochemical water splitting, and photocatalytic water splitting—have surfaced, each offering alternative solutions at various technology readiness levels.
3. Recent advancements in hydrogen production technologies
3.1. Water splitting
3.1.1. Photoelectrochemical water splitting
Photoelectrochemical (PEC) water splitting is a method that utilizes solar energy to generate hydrogen from water. This process involves the use of a semiconductor photoelectrode that absorbs solar radiation and facilitates the separation of water into oxygen and hydrogen [15]. Despite its potential for producing clean and sustainable energy, the efficiency of PEC water splitting has been a focus of research due to challenges such as the recombination of charge carriers and high overpotential [16]. Ongoing efforts are aimed at developing efficient, durable, and cost-effective PEC water splitting technologies to maximize the solar-to-hydrogen conversion efficiency.
Current research focuses on selecting suitable photoelectrode materials, such as hematite (Fe2O3), despite challenges in photochemical conversion efficiency. Hematite show promise due to their suitable energy band position, earth abundance, stability, and non-toxicity, despite facing challenges in photochemical conversion efficiency [17]. In terms of sustainability challenges and future targets, while advancements in PEC technology have garnered attention, its environmental sustainability remains uncertain. Challenges include enhancing water-splitting efficiency, minimizing production costs, and improving device durability. PEC electrodes currently fall short of the 25-year lifetime seen in commercial PV panels and electrolyzers [18]. Defining medium to long-term performance targets from an environmental perspective becomes crucial for guiding future developments in green hydrogen production.
3.1.2. Thermochemical water splitting
Thermochemical water splitting, on the other hand, involves the use of high temperatures to drive a series of chemical reactions that result in the decomposition of water into hydrogen and oxygen. This method typically relies on concentrated solar power or other high-temperature heat sources to initiate the thermochemical cycles, which are sets of chemical reactions involving intermediate compounds [19]. While photoelectrochemical water splitting is a process that directly converts solar energy into the chemical energy needed for water dissociation, thermochemical water splitting is based on high-temperature-driven chemical reactions. Unlike other water splitting methods, thermochemical water splitting is a long-term technology pathway with the potential for low or no greenhouse gas emissions [20]. The chemicals used in the process are reused within each cycle, creating a closed loop that consumes only water and produces hydrogen and oxygen. This technology is being considered due to its potential for large-scale hydrogen production with minimal environmental impact. Various thermochemical cycles have been described in the literature, with ongoing research focusing on overcoming challenges related to efficiency, durability of reactant materials, and the development of commercially viable thermochemical cycles and reactors.
Boretti et al. examines thermochemical water-splitting cycles specifically for producing hydrogen in conjunction with high-temperature concentrated solar energy, typically available at 1000 to 1100°C [21]. It emphasizes the advantageous synergy between these cycles and near-future dispatchable concentrated solar power systems, suggesting that three-step cycles offer the best compromise between working temperatures, complexity, and technological readiness. This approach benefits from past experiences in thermochemical processes for nuclear power plants and aligns well with the increasing availability of dispatchable concentrated solar power with thermal energy storage. Preliminary evaluations indicate that three-step cycles could enable cost-competitive CO2-free hydrogen production within a decade, potentially undercutting both green hydrogen from solar photovoltaic electricity and electrolysis, as well as current hydrogen production from hydrocarbons. Despite lower conversion efficiencies compared to electrolysis, the thermochemical pathway holds promise due to more efficient solar thermal energy collection and the potential for achieving a lower cost of hydrogen production.
Likewise, Sadeghi and Ghandehariun emphasized the importance of system design optimization in achieving efficiency and cost reductions. Their research explores the integration of a solar power tower system with a thermochemical water splitting cycle utilizing LiNaK high-temperature carbonate molten salt, specifically focusing on the copper-chlorine (Cu-Cl) cycle. This integrated system operates independently of external energy sources such as grid electricity or natural gas. Through comprehensive thermodynamic and economic analyses, the study evaluates system performance and hydrogen production costs. In the base case scenario, the system achieves thermal efficiencies of 40.4% for the Cu-Cl cycle, 45.74% for the supercritical steam Rankine cycle, and an overall efficiency of 28.77% [22]. The system's hydrogen production capacity is determined to be 1530.4 kg/h, with a total capital investment of $811.04 million and a levelized cost of hydrogen of $9.47/kg H2. All this data is presented in Table 2. Multi-objective optimization reveals an optimal system design with improved overall thermal efficiency (29.18%) and a reduced levelized cost of hydrogen ($7.58/kg H2). These findings highlight the potential for cost-effective hydrogen production through solar thermochemical water splitting, emphasizing the significance of system design optimization in enhancing efficiency and reducing production costs.
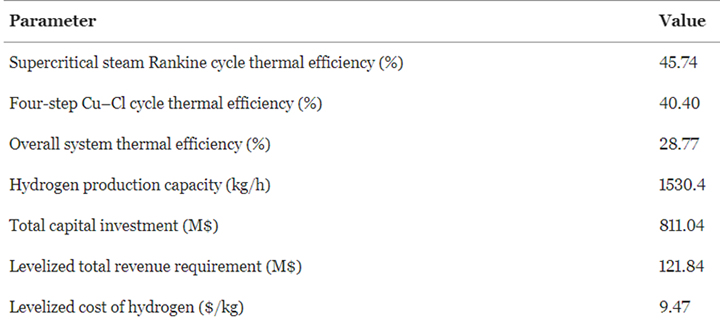
Table 2. Modeling results for the base case of this study [15].
3.1.3. Electrochemical water splitting
Electrochemical water splitting is a process that involves the use of electrical energy to decompose water into oxygen and hydrogen. This occurs through two simultaneous reactions: the hydrogen evolution reaction (HER) on the cathode, where hydrogen is produced, and the oxygen evolution reaction (OER) on the anode, where oxygen is produced [23, 24]. The process takes place in an electrolytic cell, with the anode and cathode immersed in water. Electrolysis of water is a key method for producing hydrogen and is an active area of research aimed at improving the efficiency and sustainability of the process. Recent advancements have focused on developing efficient and stable electrocatalysts to enhance the overall efficiency of water splitting, making it a promising approach for sustainable hydrogen production.
Guo et al. presents a groundbreaking method for producing high-purity hydrogen directly from atmospheric moisture through electrolysis, addressing the challenge of water scarcity in conventional hydrogen production. The direct air electrolysis (DAE) module shown in Figure 2, utilizing hygroscopic electrolytes and porous mediums, achieves remarkable performance, sustaining hydrogen generation even in bone-dry environments with relative humidity as low as 4%. With a Faradaic efficiency of approximately 95%, the DAE module operates continuously for over 12 days, demonstrating scalability and versatility for integration with renewable energy sources like solar and wind [25]. A prototype tower incorporating multiple DAE modules achieves automated hydrogen production, with potential rates reaching 3.7 m3 H2 day−1 m−2 tower on sunny days.

Figure 2. The concept of direct air electrolysis (DAE) for hydrogen production. (a) A schematic diagram of the DAE module with a water harvesting unit made of porous medium soaked with the hygroscopic ionic solution. (b) A schematic diagram of the cross-section of the DAE module, showing the electrodes are isolated from the air feed, and the absorbed water are transported to the electrode by capillaries of the sponge [25].
Another study by Al-Mahgari et al. compares various methods for producing hydrogen from water electrolysis, considering different pressure and temperature conditions. They found that increasing operating temperature reduces electrolysis work, while pressure has the opposite effect, especially at low pressures [26]. The study concludes that compressing liquid water, heating, then electrolyzing requires less energy overall than atmospheric electrolysis followed by gas compression. High-pressure steam electrolysis integrated with renewable systems is found to be a cost-effective method for producing green hydrogen, particularly with a cheap heat source or waste heat utilization.
3.1.4. Photocatalytic water splitting
Photocatalytic water splitting is a process that uses photocatalysis to harness solar energy for the dissociation of water into hydrogen and oxygen. This technology is considered a form of artificial photosynthesis, as it mimics the natural process of photosynthesis to produce a solar fuel [27]. The process requires only light energy, water, and a catalyst, similar to natural photosynthetic oxygen production. Photocatalytic water splitting can be achieved by dispersing photocatalyst particles in water or using a photoelectrochemical cell with a photoelectrode [28]. While it is an efficient technology for hydrogen production, the solar-to-hydrogen efficiency has remained relatively low [29]. However, ongoing research aims to improve the efficiency of this process and explore the potential to produce green hydrogen using solar energy.
While the solar-to-hydrogen efficiency of photocatalytic water splitting has remained low, recent developments have shown potential for significant improvement. For instance, Guo et al. proposed an efficient biphase photocatalytic system that significantly increased the efficiency of hydrogen production from water using particulate photocatalysts [30].
On the other hand, recent research by Lin et al. has explored the effectiveness of Co/Fe oxide nanoparticles as catalysts for producing hydrogen through water photolysis. The findings show that among the synthesized nanoparticles, Co3O4/Fe2O3 demonstrates the highest rate of hydrogen evolution due to the formation of a p–n heterojunction with the smallest band gap energy, which enhances light absorption and active site utilization while preventing the recombination of electron–hole pairs [31]. However, further optimization is needed, as carbon doping with graphite has been found to reduce catalytic activity.
4. Comparative analysis
The production of green hydrogen involves diverse methods, each with distinct advantages and challenges. Recent advancements emphasize the need for continuous research and development for improved device architectures and materials in hydrogen production technologies.
4.1. Raw material differences
Green hydrogen production relies on diverse raw materials, influencing efficiency, sustainability, and economic viability. Water, the feedstock for electrolysis, impacts environmental footprint and cost-effectiveness based on its source, quality, and availability. Renewable energy sources power the process, aligning hydrogen production with environmental goals. For example, using solar, wind, or hydropower has shown promising results in terms of sustainability and clean energy integration. Additionally, alternative feedstocks like biomass, natural gas, coal, and industrial waste pose both challenges and opportunities for sustainability.
Biomass presents a renewable pathway for hydrogen generation, but efficient conversion technologies are needed to overcome competition with food production. Research is actively seeking sustainable biomass sources and refining conversion processes to enhance its feasibility as a hydrogen feedstock [32]. Natural gas, while commonly used, raises carbon emission concerns, yet integration with carbon capture, utilization, and storage (CCUS) technologies can mitigate its environmental impact. Renewable natural gas from bio- and waste-based sources offers a low-carbon or decarbonized hydrogen option, aligning with sustainability objectives [33]. Coal, though emissions-intensive, is under scrutiny for hydrogen production, with ongoing research focusing on reducing environmental impact through advanced carbon capture and utilization techniques. Meanwhile, industrial waste presents an opportunity for hydrogen production, but its efficient utilization requires technological innovation and rigorous waste management practices to ensure compliance with environmental and safety standards [34]. These alternative feedstocks offer diverse pathways for green hydrogen production, and addressing the associated challenges while maximizing the opportunities for sustainability and economic viability requires ongoing research, technological innovation, and stringent environmental management practices.
In terms of challenges, cost considerations and the economic landscape significantly influence the viability of hydrogen production methods. While the cost of green hydrogen historically exceeded that of gray hydrogen from fossil fuels, recent trends show competitive pricing. For instance, the cost of gray hydrogen produced from natural gas is currently between 5.5 and 6.89 USD/kg in various regions, while green hydrogen ranges between 4.84 and 6.68 USD/kg. Policy shifts, as seen in South Korea, indicate a move towards large-scale green hydrogen production from renewable sources by 2040, with a targeted decrease in costs to 2.33 USD/kg by 2040 [35].
4.2 Geographic Variances
Countries' approaches to green hydrogen technologies vary significantly based on their unique energy landscapes, economic conditions, and policy priorities. For instance, the green hydrogen national targets of Germany, France, Portugal, the Netherlands, and Spain already account for more than 50 percent of the total targets set by the European Union. These countries have set ambitious goals and have highly diversified strategies and focus areas, reflecting their individual priorities and capabilities [36].
Furthermore, the Asia-Pacific region comprises a number of energy importers, technology exporters, and countries with strong energy export potential due to ideal settings for green hydrogen production [37]. This has led to ambitious goals frequently compared to those of the European Union, demonstrating the diverse approaches taken by countries in the region to embrace green hydrogen as a key element in their energy transition.
Geopolitical and geo-economic factors also play a significant role in shaping national hydrogen strategies. The local availability and ease of access to hydrogen are key determinants in a country's hydrogen strategy. For example, countries with an abundance of low-cost renewable power could become producers of green hydrogen, with commensurate geoeconomic and geopolitical consequences. This illustrates how a country's unique energy landscape and industrialization influence its approach to green hydrogen technologies [38].
Countries around the world are embracing green hydrogen as an essential element in their energy transition, implementing diverse strategies to integrate this sustainable energy source. Notable initiatives include the World Bank Group's efforts, Australia's National Hydrogen Strategy, India's Green Hydrogen Policy, Japan's comprehensive global hydrogen supply chain, Bangladesh's foray into hydrogen energy, Germany's cutting-edge hydrogen policy, and China's strategic hydrogen energy development plan [39].
5. Conclusion
This review highlights the importance green hydrogen in addressing the global energy crisis and combating climate change. As nations strive to meet their carbon neutrality targets and transition towards sustainable energy sources, green hydrogen emerges as a promising solution due to its versatility, zero-emission profile, and potential for large-scale production.
Advancements in green hydrogen production technologies, particularly in electrolysis and water splitting methods, demonstrate ongoing efforts to enhance efficiency, reduce costs, and improve sustainability. Research endeavors are focused on overcoming technical challenges and optimizing systems for commercial viability, spanning from photoelectrochemical and thermochemical water splitting to electrochemical processes.
Furthermore, the comparative analysis reveals the importance of raw materials and geographic variances in the field of green hydrogen production. While water remains a primary feedstock, alternative sources such as biomass, natural gas, coal, and industrial waste present both challenges and opportunities for sustainability. Moreover, countries worldwide exhibit diverse approaches to green hydrogen adoption, driven by unique energy landscapes, economic conditions, and policy priorities.
Looking ahead, the outlook for green hydrogen appears promising but requires concerted efforts across multiple fronts. Technological innovation, policy support, infrastructure development, and international collaboration are crucial for unlocking the full potential of green hydrogen and realizing a sustainable energy future. As costs continue to decline, and scalability improves, green hydrogen is poised to play a central role in global efforts to mitigate climate change and secure reliable, clean energy supplies for generations to come.
 Dr. Raj Shah serves in the role of Director at Koehler Instrument Company in New York, boasting an impressive 28-year tenure with the organization. Recognized as a Fellow by eminent organizations such as IChemE, CMI, STLE, AIC, NLGI, INSTMC, Institute of Physics, The Energy Institute, and The Royal Society of Chemistry, he stands as a distinguished recipient of the ASTM Eagle award. Dr. Shah, a luminary in the field, recently coedited the highly acclaimed "Fuels and Lubricants Handbook," a bestseller that unravels industry insights. Explore the intricacies at ASTM's Long-Awaited Fuels and Lubricants Handbook 2nd Edition Now Available (https://bit.ly/3u2e6GY).
Dr. Raj Shah serves in the role of Director at Koehler Instrument Company in New York, boasting an impressive 28-year tenure with the organization. Recognized as a Fellow by eminent organizations such as IChemE, CMI, STLE, AIC, NLGI, INSTMC, Institute of Physics, The Energy Institute, and The Royal Society of Chemistry, he stands as a distinguished recipient of the ASTM Eagle award. Dr. Shah, a luminary in the field, recently coedited the highly acclaimed "Fuels and Lubricants Handbook," a bestseller that unravels industry insights. Explore the intricacies at ASTM's Long-Awaited Fuels and Lubricants Handbook 2nd Edition Now Available (https://bit.ly/3u2e6GY).
His academic journey includes a doctorate in Chemical Engineering from The Pennsylvania State University, complemented by the title of Fellow from The Chartered Management Institute, London. Dr. Shah holds the esteemed status of a Chartered Scientist with the Science Council, a Chartered Petroleum Engineer with the Energy Institute, and a Chartered Engineer with the Engineering Council, UK. Recently honored as "Eminent Engineer" by Tau Beta Pi, the largest engineering society in the USA, Dr. Shah serves on the Advisory Board of Directors at Farmingdale University (Mechanical Technology), Auburn University (Tribology), SUNY Farmingdale (Engineering Management), and the State University of NY, Stony Brook (Chemical Engineering/Material Science and Engineering).
In tandem with his role as an Adjunct Professor at the State University of New York, Stony Brook, in the Department of Material Science and Chemical Engineering, Dr. Shah's impact spans over three decades in the energy industry, with a prolific portfolio of over 600 publications. Dive deeper into Dr. Raj Shah's journey at https://bit.ly/3QvfaLX.
For further correspondence, reach out to Dr. Shah at rshah@koehlerinstrument.com.
Simultaneously, within the dynamic internship program at Koehler Instrument Company in Holtsville, Beau Eng, William Streiber, and Gavin S. Thomas emerge as standout participants. These aspiring talents, all pursuing studies in Chemical Engineering at Stony Brook University, Long Island, NY, thrive under the mentorship of Dr. Raj Shah, the current Chairman of the External Advisory Board of Directors at the university.
References
[1] U. Nations. "Causes and Effects of Climate Change." United Nations. https://www.un.org/en/climatechange/science/causes-effects-climate-change (accessed.
[2] P. Sands, "The United Nations framework convention on climate change," Rev. Eur. Comp. & Int'l Envtl. L., vol. 1, p. 270, 1992.
[3] I. E. Agency, "Hydrogen," 2023. [Online]. Available: https://www.iea.org/energy-system/low-emission-fuels/hydrogen.
[4] A. A. Collera, "Opportunities for production and utilization of green hydrogen in the Philippines," International Journal of Energy Economics and Policy, 2021.
[5] W. E. Council, "International hydrogen strategies," 2022. [Online]. Available: https://www.weltenergierat.de/publikationen/studien/international-hydrogen-strategies/.
[6] E. Commission, "2050 long-term strategy," 2022. [Online]. Available: https://climate.ec.europa.eu/eu-action/climate-strategies-targets/2050-long-term-strategy_en.
[7] N. Grid, "The hydrogen colour spectrum," 2022. [Online]. Available: https://www.nationalgrid.com/stories/energy-explained/hydrogen-colour-spectrum.
[8] I. E. Agency. "Global Hydrogen Review 2021 – Analysis." https://www.iea.org/reports/global-hydrogen-review-2021 (accessed.
[9] IEA, "Towards hydrogen definitions based on their emissions intensity," IEA, 2023. [Online]. Available: https://www.iea.org/reports/towards-hydrogen-definitions-based-on-their-emissions-intensity.
[10] B. S. Zainal et al., "Recent advancement and assessment of green hydrogen production technologies," Renewable and Sustainable Energy Reviews, vol. 189, p. 113941, 2024/01/01/ 2024, doi: https://doi.org/10.1016/j.rser.2023.113941.
[11] S. Calnan et al., "Development of Various Photovoltaic-Driven Water Electrolysis Technologies for Green Solar Hydrogen Generation," Solar RRL, vol. 6, no. 5, p. 2100479, 2022, doi: https://doi.org/10.1002/solr.202100479.
[12] L. Cattani, P. Cattani, A. Magrini, R. Figoni, D. Dondi, and D. Vadivel, "Suitability and Energy Sustainability of Atmospheric Water Generation Technology for Green Hydrogen Production," (in English), Energies, Article vol. 16, p. NA, 2023/09//
// 2023. [Online]. Available: https://link-gale-com.proxy.library.stonybrook.edu/apps/doc/A771810435/AONE?u=sunysb&sid=bookmark-AONE&xid=3e62bc1c.
[13] M. El-Shafie, S. Kambara, and Y. Hayakawa, "Hydrogen production technologies overview," 2019.
[14] H. A. Yousefi Rizi and D. Shin, "Green Hydrogen Production Technologies from Ammonia Cracking," Energies, vol. 15, no. 21, p. 8246, 2022. [Online]. Available: https://www.mdpi.com/1996-1073/15/21/8246.
[15] M. Kumar, B. Meena, P. Subramanyam, D. Suryakala, and C. Subrahmanyam, "Recent trends in photoelectrochemical water splitting: the role of cocatalysts," NPG Asia Materials, vol. 14, no. 1, p. 88, 2022/11/11 2022, doi: 10.1038/s41427-022-00436-x.
[16] J. M. Yu et al., "High-performance and stable photoelectrochemical water splitting cell with organic-photoactive-layer-based photoanode," Nature Communications, vol. 11, no. 1, p. 5509, 2020/11/02 2020, doi: 10.1038/s41467-020-19329-0.
[17] Z. Najaf et al., "Recent trends in development of hematite (α-Fe2O3) as an efficient photoanode for enhancement of photoelectrochemical hydrogen production by solar water splitting," International Journal of Hydrogen Energy, vol. 46, no. 45, pp. 23334-23357, 2021/07/01/ 2021, doi: https://doi.org/10.1016/j.ijhydene.2020.07.111.
[18] G. Segev et al., "The 2022 solar fuels roadmap," Journal of Physics D: Applied Physics, vol. 55, no. 32, p. 323003, 2022/06/22 2022, doi: 10.1088/1361-6463/ac6f97.
[19] V. K. Budama, J. P. Rincon Duarte, M. Roeb, and C. Sattler, "Potential of solar thermochemical water-splitting cycles: A review," Solar Energy, vol. 249, pp. 353-366, 2023/01/01/ 2023, doi: https://doi.org/10.1016/j.solener.2022.11.001.
[20] F. Safari and I. Dincer, "A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production," Energy Conversion and Management, vol. 205, p. 112182, 2020/02/01/ 2020, doi: https://doi.org/10.1016/j.enconman.2019.112182.
[21] A. Boretti, "Which thermochemical water-splitting cycle is more suitable for high-temperature concentrated solar energy?," International Journal of Hydrogen Energy, vol. 47, no. 47, pp. 20462-20474, 2022/06/01/ 2022, doi: https://doi.org/10.1016/j.ijhydene.2022.04.159.
[22] S. Sadeghi and S. Ghandehariun, "A standalone solar thermochemical water splitting hydrogen plant with high-temperature molten salt: Thermodynamic and economic analyses and multi-objective optimization," Energy, vol. 240, p. 122723, 2022/02/01/ 2022, doi: https://doi.org/10.1016/j.energy.2021.122723.
[23] H. Sun, X. Xu, Y. Song, W. Zhou, and Z. Shao, "Designing High-Valence Metal Sites for Electrochemical Water Splitting," Advanced Functional Materials, vol. 31, no. 16, p. 2009779, 2021, doi: https://doi.org/10.1002/adfm.202009779.
[24] J. Wang et al., "Non-precious-metal catalysts for alkaline water electrolysis: operando characterizations, theoretical calculations, and recent advances," Chemical Society Reviews, 10.1039/D0CS00575D vol. 49, no. 24, pp. 9154-9196, 2020, doi: 10.1039/D0CS00575D.
[25] J. Guo et al., "Hydrogen production from the air," Nature Communications, vol. 13, no. 1, p. 5046, 2022/09/06 2022, doi: 10.1038/s41467-022-32652-y.
[26] A. M. Al-Mahgari, M. d. A. Al-Nimr, and S. A. Khashan, "Pressurized green hydrogen from water electrolysis: Compression before or after electrolysis? A comparison among different configurations," Journal of Energy Storage, vol. 73, p. 109251, 2023/12/20/ 2023, doi: https://doi.org/10.1016/j.est.2023.109251.
[27] R. Li and C. Li, "Chapter One - Photocatalytic Water Splitting on Semiconductor-Based Photocatalysts," in Advances in Catalysis, vol. 60, C. Song Ed.: Academic Press, 2017, pp. 1-57.
[28] A. Hakki, Y. AlSalka, C. B. Mendive, J. Ubogui, P. C. dos Santos Claro, and D. Bahnemann, "Hydrogen Production by Heterogeneous Photocatalysis," in Encyclopedia of Interfacial Chemistry, K. Wandelt Ed. Oxford: Elsevier, 2018, pp. 413-419.
[29] K. Takanabe, "Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design," ACS Catalysis, vol. 7, no. 11, pp. 8006-8022, 2017/11/03 2017, doi: 10.1021/acscatal.7b02662.
[30] S. Guo, X. Li, J. Li, and B. Wei, "Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems," Nature Communications, vol. 12, no. 1, p. 1343, 2021/02/26 2021, doi: 10.1038/s41467-021-21526-4.
[31] S. Lin and T. Zhang, "Active Co/Fe composite oxide nanoparticles for efficient photocatalytic hydrogen production," Journal of Materials Science: Materials in Electronics, vol. 33, no. 16, pp. 13444-13453, 2022/06/01 2022, doi: 10.1007/s10854-022-08281-y.
[32] L. Cao et al., "Biorenewable hydrogen production through biomass gasification: A review and future prospects," Environmental Research, vol. 186, p. 109547, 2020/07/01/ 2020, doi: https://doi.org/10.1016/j.envres.2020.109547.
[33] K. A. Davis, S. Yoo, E. W. Shuler, B. D. Sherman, S. Lee, and G. Leem, "Photocatalytic hydrogen evolution from biomass conversion," Nano Convergence, vol. 8, no. 1, p. 6, 2021/02/26 2021, doi: 10.1186/s40580-021-00256-9.
[34] V. G. Nguyen et al., "Recent advances in hydrogen production from biomass waste with a focus on pyrolysis and gasification," International Journal of Hydrogen Energy, vol. 54, pp. 127-160, 2024/02/07/ 2024, doi: https://doi.org/10.1016/j.ijhydene.2023.05.049.
[35] C. Park, M. Koo, J. Woo, B. I. Hong, and J. Shin, "Economic valuation of green hydrogen charging compared to gray hydrogen charging: The case of South Korea," International Journal of Hydrogen Energy, vol. 47, no. 32, pp. 14393-14403, 2022/04/15/ 2022, doi: https://doi.org/10.1016/j.ijhydene.2022.02.214.
[36] V. P. Müller and W. Eichhammer, "Economic complexity of green hydrogen production technologies - a trade data-based analysis of country-specific industrial preconditions," Renewable and Sustainable Energy Reviews, vol. 182, p. 113304, 2023/08/01/ 2023, doi: https://doi.org/10.1016/j.rser.2023.113304.
[37] W. Cheng and S. Lee, "How Green Are the National Hydrogen Strategies?," Sustainability, vol. 14, no. 3, p. 1930, 2022. [Online]. Available: https://www.mdpi.com/2071-1050/14/3/1930.
[38] T. Van de Graaf, I. Overland, D. Scholten, and K. Westphal, "The new oil? The geopolitics and international governance of hydrogen," Energy Research & Social Science, vol. 70, p. 101667, 2020/12/01/ 2020, doi: https://doi.org/10.1016/j.erss.2020.101667.
[39] T. Gupta, S. Raha, K. Sawant, and A. Pornet, "Indo-Pacific Energy Transition."
The content & opinions in this article are the author’s and do not necessarily represent the views of AltEnergyMag
Comments (0)
This post does not have any comments. Be the first to leave a comment below.
Featured Product

